Hearing Aids and PSAPs: Making the Choice
Today’s aging population is fueling a growing demand for affordable hearing aids and alternative hearing devices. As the types of these devices multiply, consumers are faced with a confusing array of choices. Millions are wondering, “If I have mild-to-moderate hearing loss, what is my best choice?”
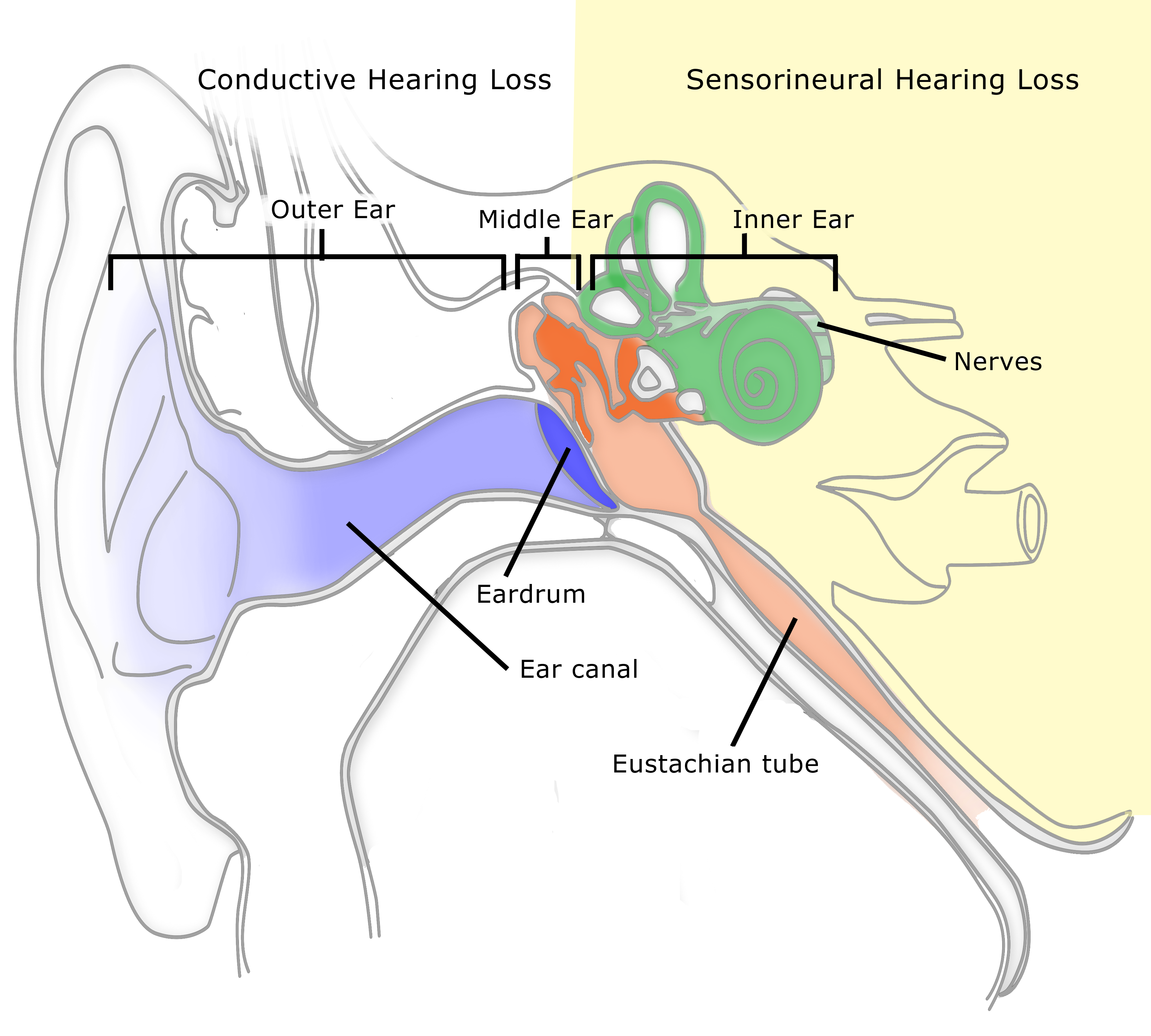
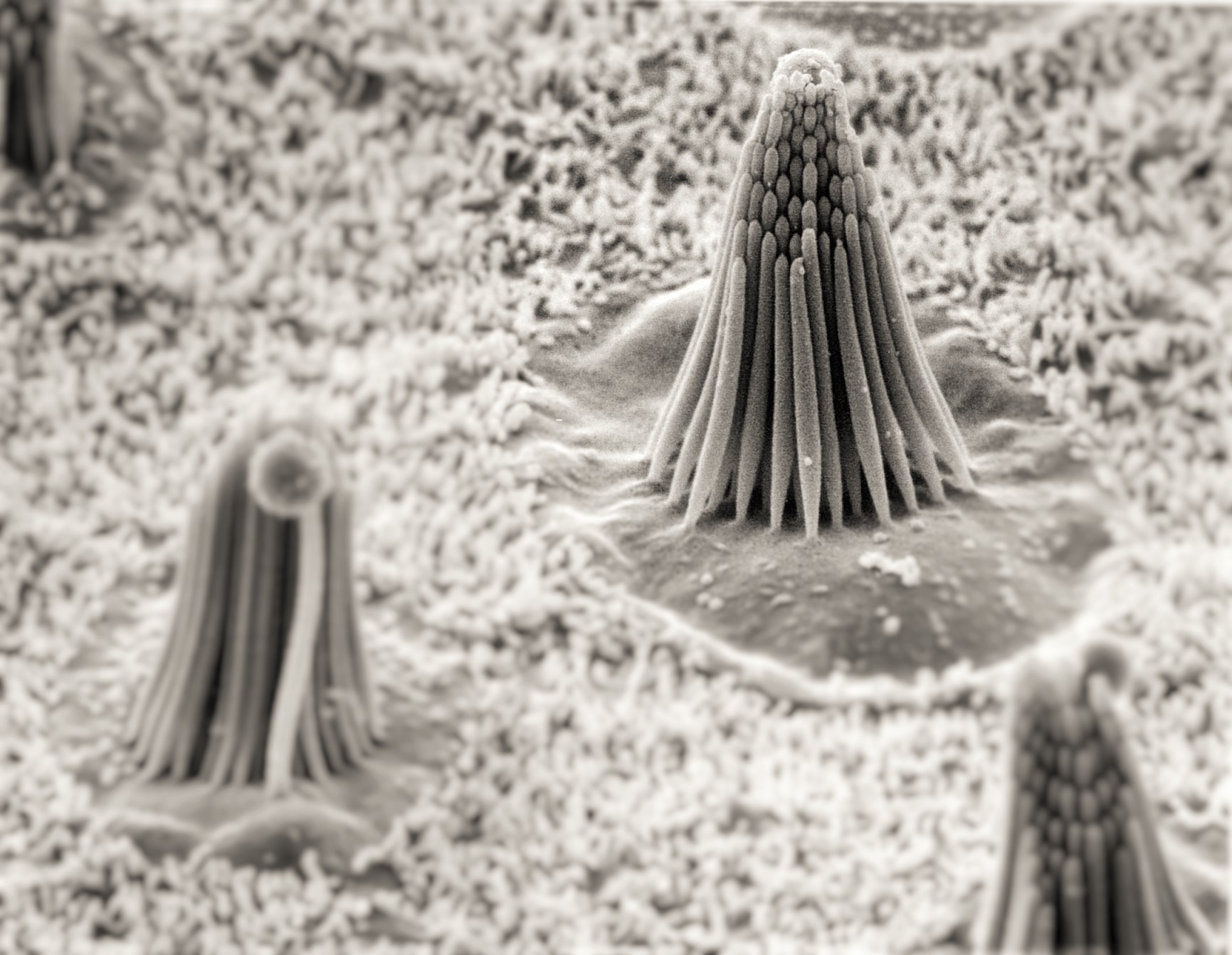
 By now you have probably heard about one kind of wearable hearing product: a personal sound amplification device or PSAP (occasionally referred to as an assistive listening device or ALD). PSAPs have a limited function compared to modern programmable hearing aids. They are not subject to Food and Drug Administration (FDA) requirements, testing, and regulations like those imposed on hearing aids. This means claims made about them are largely unproven. They are available over the counter and on line, and thus not fitted to the customer as hearing aids can be. On the other hand, PSAPs are available at a fraction of the cost of hearing aids.
By now you have probably heard about one kind of wearable hearing product: a personal sound amplification device or PSAP (occasionally referred to as an assistive listening device or ALD). PSAPs have a limited function compared to modern programmable hearing aids. They are not subject to Food and Drug Administration (FDA) requirements, testing, and regulations like those imposed on hearing aids. This means claims made about them are largely unproven. They are available over the counter and on line, and thus not fitted to the customer as hearing aids can be. On the other hand, PSAPs are available at a fraction of the cost of hearing aids.
Q: In layman’s terms, what is the difference between hearing aids and PSAPs?
A: The FDA employs the intended use of the two devices to distinguish between them and to classify them as medical devices or simply electronic products.
The FDA says hearing aids are appropriately used for difficulty:
- Hearing a person nearby
- Hearing conversation in crowded rooms
- Understanding movie dialog in a theater
- Listening to lectures in a quiet room
- Hearing the ring of a doorbell or phone
- Understanding speech where environmental noise can interfere
PSAPs, on the other hand, are considered by the FDA to be useful:
- Hearing a distant lecturer or conversation
- Listening for prey while hunting
- Hearing bird sounds
- Listening to soft sounds that normal hearers would find difficult to hear
Q: Has the FDA created an official definition of PSAPs and hearing aids?
A: Yes. In a 2013 update, the FDA defined a hearing aid as “a wearable sound-amplifying device that is intended to compensate for impaired hearing.” Hearing aid features include:
- Programmability “to address an individual’s degree of hearing loss across sound frequencies to improve speech intelligibility”
- Possible acoustical or wireless coupling to “external electronic products such as televisions, MP3 players, and telephones”
- Fitting by an audiologist, hearing aid dispenser or another hearing health professional to “program and optimize” hearing aid performance with its “more complex features”
In the same document, a PSAP was described as a “wearable electronic product that is not intended to compensate for impaired hearing.” PSAPs:
- Are intended for use by non-hearing-impaired people to amplify sounds during activities like hunting or other recreational activities
- Are usually “simpler sound amplification devices with fewer features and less functionality than hearing aids”
- May nonetheless share “some of the technology and functionality” of hearing aids 1
Q: Has the FDA taken a position for or against PSAPs?
A: Not specifically. However, according to Eric Mann, M.D., Ph.D., clinical deputy director for the FDA Division of Ophthalmic, and Ear, Nose, and Throat Devices, “Consumers should not confuse hearing aids with personal sound amplification products (PSAPs.) Although some PSAP technology is similar to that of a hearing aid, only hearing aids are intended to make up for impaired hearing.”
“FDA regulates hearing aids as medical devices in order to assure their safety and effectiveness. PSAPs are not subject to medical device regulations, although they are subject to other safety regulations as an electronic product that emits sound vibrations.” 2
A Final Word for Consumers
As you might expect, representatives of the hearing aid industry emphasize the capabilities and features that PSAPs lack. At the same time, ads and testimonials for PSAPs claim their effective use by people with mild-to-moderate hearing loss and point out their extremely low cost compared to that of hearing aids.
If you have mild-to-moderate hearing loss or difficulty hearing in certain circumstances and have consulted an audiologist or physician specializing in hearing loss to rule out other ear problems, you may want to try a PSAP. Before you buy one, make sure you understand the store’s or web site’s return policy. If you purchase a PSAP whose manufacturer claims it can be used to assist the hearing impaired, remember that the FDA does not consider PSAPs to be medical devices and has not issued standards for them.
Another affordable approach to dealing with mild-to-moderate hearing loss is purchasing external equipment that targets the circumstances where you notice the problem. You may have seen advertisements for devices that let you enjoy the TV and musical equipment through earphones. There is even an app that turns your smartphone into an amplifier when you use it with earbuds.
References
1http://www.fda.gov/forconsumers/consumerupdates/ucm372926.htm
2http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm373461.htm
For More Information
http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm532005.htm
http://www.nationalacademies.org/hmd/Reports/2016/Hearing-Health-Care-for-Adults.aspx
http://www.hearingreview.com/2016/11/senators-warren-grassley-introduce-otc-hearing-aid-act-2016/




















Leave a Reply